您想继续阅读英文文章还
是切换到中文?
是切换到中文?

THINK ALUMINIUM THINK AL CIRCLE

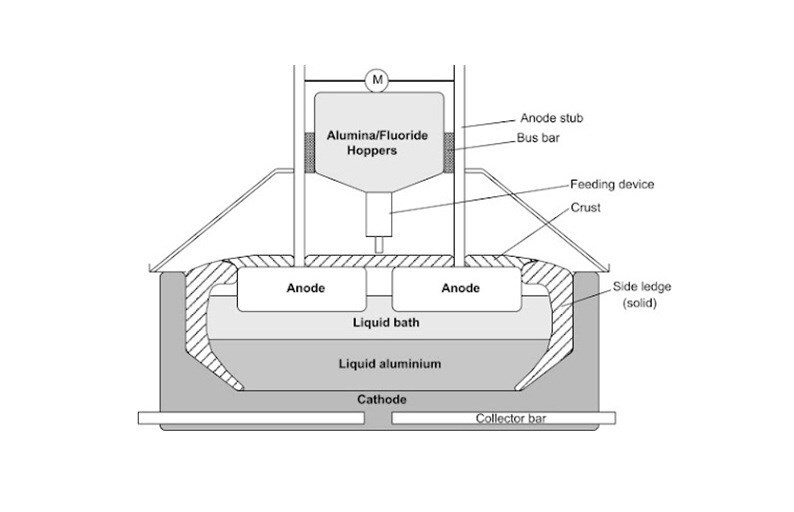
Aluminium fluoride (AlF3) has long been a critical ingredient in aluminium production – vital for efficient electrolysis yet scrutinised for its environmental and economic costs. On one hand, it is a necessity, on the other, a dilemma. Functionally, aluminium fluoride is a cornerstone of the Hall–Héroult process as it reduces electrolyte’s melting point from ~2000°C to <1000°C, cuts energy consumption, increases the bath’s conductivity, and helps dissolve alumina into the electrolyte. In short, this additive allows for efficient electrolysis of alumina (Al₂O₃) into molten aluminum metal.
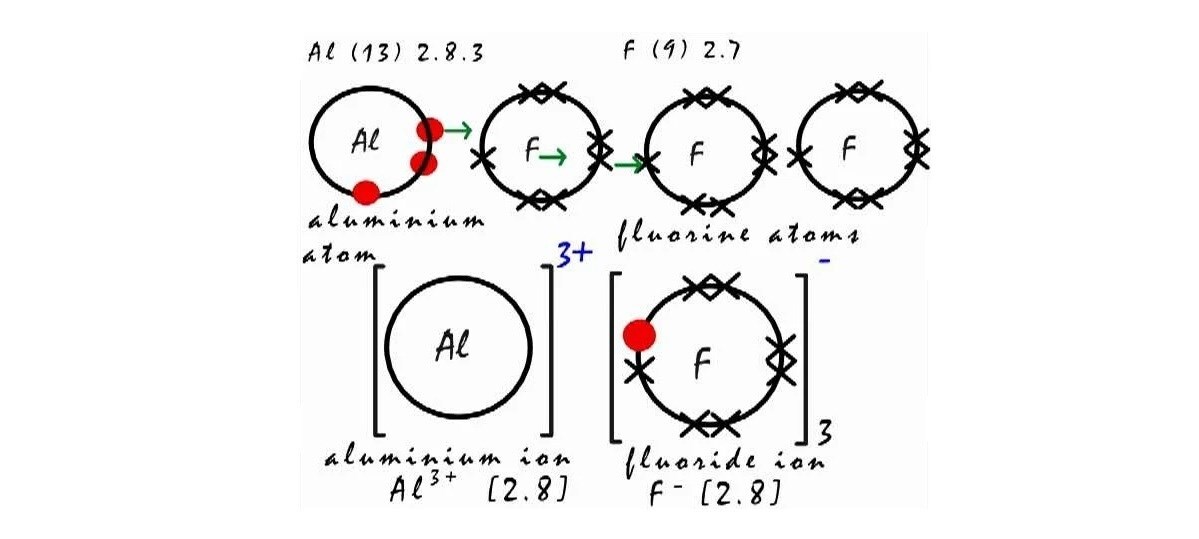
But its utility comes at a cost, both literally and environmentally. The price of aluminium fluoride, although varies by purity and supplier, typically ranges between USD 1,000 and USD 1,800 per tonne in China for industrial grades (99 per cent purity). Globally (ex-China), the price ranges around USD 1,200-1,700 per tonne. Like mentioned above, in addition to the high costs it bears, the raw material carries a tag of high GHG emitter. Well, aluminium fluoride itself in not the main emitter of greenhouse gas emissions in smelting, but of course, the process does. When aluminium fluoride is added and alumina levels drop in the electrolyte bath which is called “anode effect”, voltage increases causing carbon from the anode react with the fluorine from the dissociated cryolite bath (which contains AlF3), leading to potent perfluorocarbon (PFC) gas emissions. The primary PFCs emitted are tetrafluoromethane (CF4) and hexafluoroethane (C2F6), which are two extremely potent greenhouse gases with long-term atmospheric impacts.
Now, in this context, the question arises is whether this additive can be avoided or replaced in the electrolysis process? Likely not because aluminium fluoride fulfils multiple interconnected chemical and operational roles that other additives have yet to replicate at industrial scale. In smelting lines that run continuously and require precise bath chemistry, aluminium fluoride remains irreplaceable.
…and so much more!
SIGN UP / LOGINResponses








