您想继续阅读英文文章还
是切换到中文?
是切换到中文?

THINK ALUMINIUM THINK AL CIRCLE

In the 19th century, steel became the backbone of global industrial strategy. In the 20th century, it was oil. And, in the 21st century, it is the rare earth elements. 17 metallic elements from the periodic table, chemically similar and physically unremarkable to the eye, have become the scaffolding of electric vehicles, wind turbines, fighter jets, smartphones, navigation systems, medical devices, modern weapons and everything else that powers clean energy, digital connectivity and national defence. They are the ingredients behind the magnets that spin a wind turbine’s rotor, the catalysts that refine fuels, the phosphors that light an aircraft cockpit, and the polishing compounds that perfect wafer-thin lenses.
Explore- Most accurate data to drive business decisions with 50+ reports across the value chain
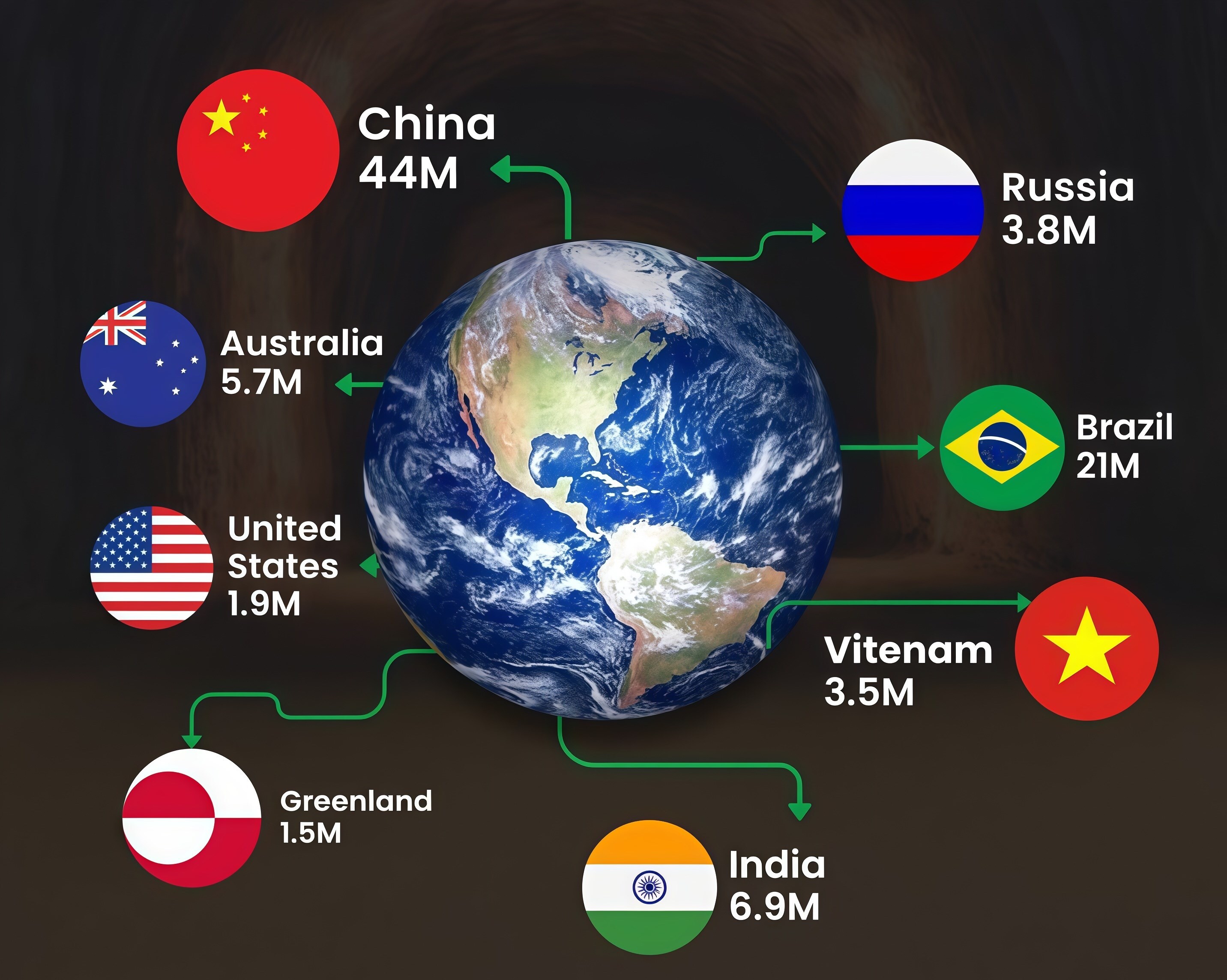
Why are these named ‘rare earth’?
The very name “rare earth” is misleading. These metals are not rare in absolute geological terms. Cerium, for instance, is more abundant in the Earth’s crust than copper. What makes them “rare” is the economic viability of extraction and processing. They seldom appear in concentrated ore bodies that are easy to dig and refine.
They are dispersed, chemically entangled with other minerals, or locked inside sands.
To complicate matters further, monazite and other common rare-earth minerals carry thorium or uranium, and the environmental equation becomes even more fraught. Strict radiation-handling regimes slow projects; lax regimes leave behind tailings ponds and anxious communities.
…and so much more!
SIGN UP / LOGINResponses








